Targeted Pills for IBD with Lower Dose, Fewer Side Effects
GI-directed Immunomodulatory Therapeutics
Tags: Monash University, Australia, Healthcare & Lifesciences
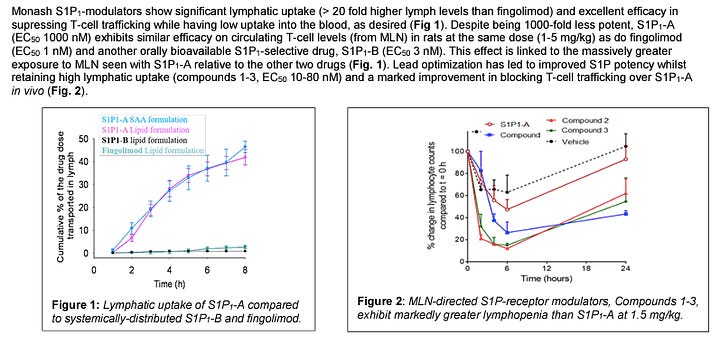
This innovation introduces GI-selective S1P1-modulators for targeting the mesenteric lymph node to treat inflammatory bowel diseases (IBD) via oral administration. It aims to decrease dosage requirements while minimizing systemic exposure, offering a GI-specific immunosuppression method. The advanced leads have nanomolar potency and demonstrate efficacy in vivo, significantly reducing T-cell trafficking. This approach circumvents the cardiovascular and non-specific immunosuppressive side effects associated with existing treatments. It presents a promising therapeutic option for IBD, with potential for broader immunomodulatory applications.
IP Type or Form Factor: Patent Pending; Platform
TRL: 5 - prototype ready for testing in intended environment
Industry or Tech Area: Pharmaceutical Engineering; Healthcare Provider